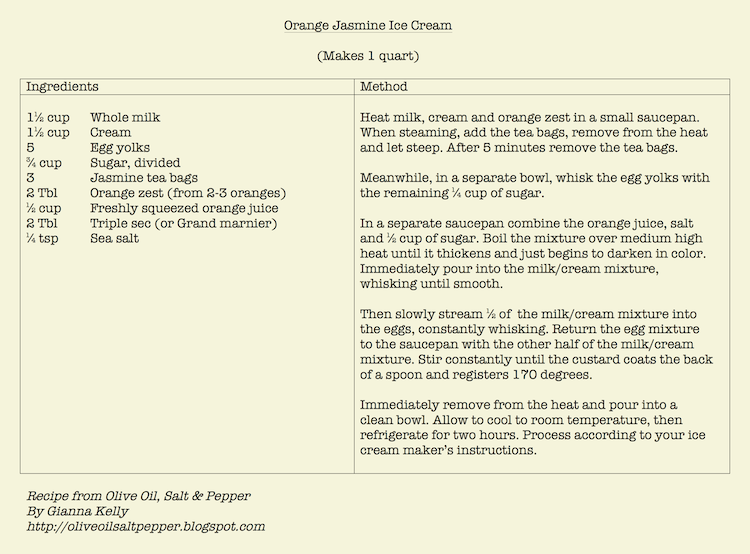
 |
| Jasmine Orange Ice Cream |
A perfect example of what I wrote about previously, this recipe was created because a bunch of beautiful local oranges fell into my lap, so to speak. We were at our good friends' house this past weekend, and they had this amazing orange tree, loaded with oranges. And they asked me what the heck they should do with all those oranges ...
So I googled how to use oranges, because, well, I'd had a glass or two of wine, and we were watching football, and I wasn't feeling particularly creative at that moment. I found this, and this, and then I found this recipe for jasmine orange cupcakes. Jasmine and orange is a brilliant combination, and not only because the smell of jasmine reminds me a lot of the scent of orange blossoms.
 |
| Orange blossoms and jasmine blossom. Images available at: http://tinyurl.com/5qbx6 |
I let that jasmine-orange idea sit in my head overnight. And then I thought . . . ice cream! Aside from emotional chemistry, there is quiet a bit of actual chemistry involved in this (and all) ice cream making. Frankly, ice cream is a rather fantastic structure of air, ice, fat, and sugar.
 |
| Ice cream under the microscope (From Clarke, 2012, “The Science of Ice Cream” Physics Education 38 (3)) |
There is a fun article on the science of ice cream at Ice Cream Nation if you'd like to read more. And if you'd rather just eat ice cream instead . . .
No comments:
Post a Comment